- FR
- EN
Cathodic protection systems
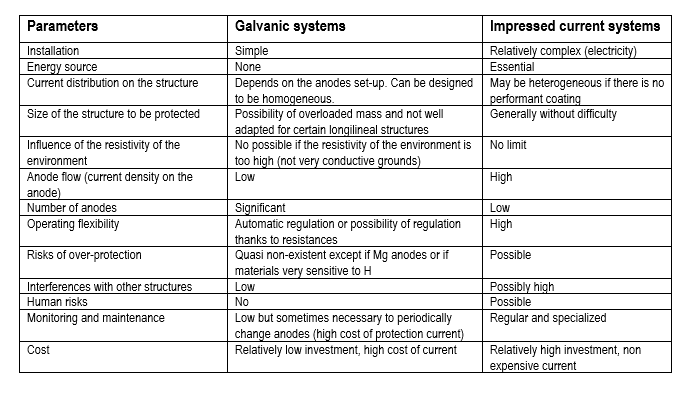
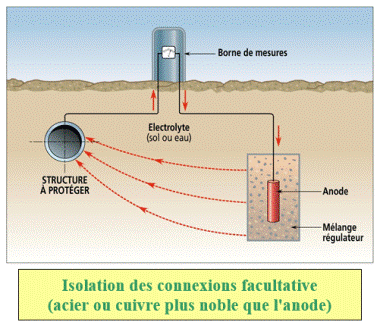
Galvanic cathodic protection
Some metals or alloys placed in the same electrolytic environment as the structure to be protected have an electrode potential (measured with regard to a reference electrode) lower than the one of the metal of the structure. When such a metal is electrically connected to the structure to be protected, its potential tends to increase, whereas the one of the metal of the structure tends to decrease, which results in the decrease of its corrosion rate. This method is limited to the electrolytes which have a resistivity that is not very high.
According to the situation, the galvanic anodes (sometimes called sacrificial or consumable anodes) are made with magnesium, zinc or aluminium. In all cases the anodes for cathodic protection must imperatively be made of specific alloys which were first qualified by measurements that enable to know their electrochemical characteristics, in particular their reactivity and their polarizability, on long term.

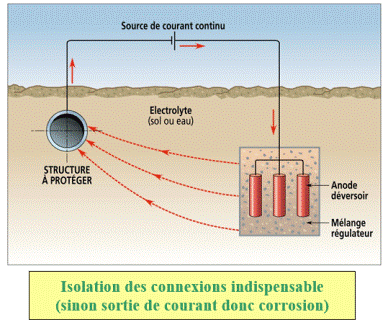
Protection by impressed current
In the cathodic protection installations by impressed current, the power supply enables to cathodically polarize the structure to be protected with a direct current that flows between the structure to be protected and an auxiliary anode called " groundbed ". In the isolated areas, this current can be generated by wind mills or photovoltaic panels systems.
According to the situation, this anode can be made of consumable material (scrap steel), that can be semi-inert (ferro-silicon, graphite, magnetite, lead oxide) or inert (platinized titanium, platinized niobium, platinized tantalum, titanium covered with mixed oxides " Ti/MMO ", conducting polymers).