Cathodic protection efficiency criteria
Level of protection
The corrorsion rate of a metallic material, expressed for example in mm/an, is proportional to the anodic current density, in A/m 2 (according to the Faraday's law). For carbon steel, the equivalence is close to 1mm/year <--> 7.8 kg/m 2 year <--> 850 mA/m 2 . When a cathodic protection installation is designed, it is necessary to make sure that in any point of the structure, the corrosion will be sufficiently reduced by the reduction of potential. The criterion generally selected is the one leading to a residual corrosion rate lower than 10 μm/year.
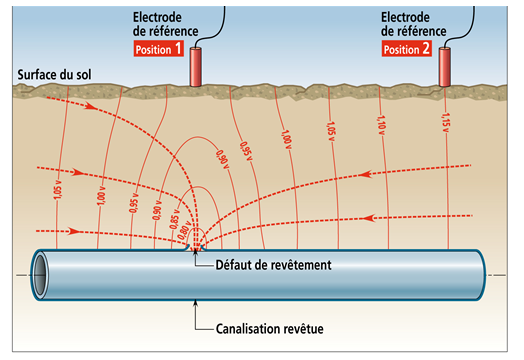
The efficiency criteria we need to consider for a cathodic protection installation, when it works, are founded on the corrosion potential of steel with regard to a reference electrode. The method to measure this potential depends on the type of structure and on the environment which surrounds it. It should be noted that the value of the ohmic fall (coming from the current intensity produced by the electric resistance) existing between the measurement electrode and the surface for which we wish to measure the potential can lead to significant measurement errors. In low-conductive environments like grounds, it is necessary to eliminate or reduce as much as we can this ohmic fall either by placing the measurement electrode in the immediate vicinity of the surface of metal, or, most often, by measuring the potential right after the interruption of cathodic protection current, in case of absence of external electric disturbances.

Influence of the ohmic fall on the measure of the électrode potential of a pipe
(Even though the position 1 for the reference electrode is better than the position 2, the measurement is distorted by the ohmic fall)
From experience, in the usual aqueous environments, a cathodic protection is efficient if the real potential of steel (except ohmic fall) is lower than –850 mV with regard to a copper/saturated copper sulphate electrode or –800 mV with regard to a silver/silver chloride/sea water electrode. In some cases, for example under anaerobic corrosion conditions (mud in sea-bed for example), the potential must be still lowered by 100 mV (that is to say respectively –950 mV or –900 mV).
Other more or less empirical criteria are sometimes took into account. The most wide-spread one is the one called " depolarization ": the potential of steel is measured as quickly as possible after the cathodic protection current is switch off (so, we avoid the ohmic fall), then a few hours afterwards. The protection is considered effective if the corrosion potential of the protected structure is at least 100 mV higher.
Cathodic over-protection risks
The potential of steel under cathodic protection should not be lowered too much (or, which is equivalent, the cathodic current density should not be too high). Indeed, in this case, some hydrogen can be formed (risk of embrittlement of certain steels) and the pH can become very alkaline, (coating damage). Some criteria linked to the risks of over-protection appear in the technical standards and recommendations.

