- FR
- EN
Cathodic protection design- immersed structures
Cathodic protection by impressed current is only used on boats hulls and fixed structures, whereas galvanic anodes are usually used for all types of structures and are fixed directly on those ones.
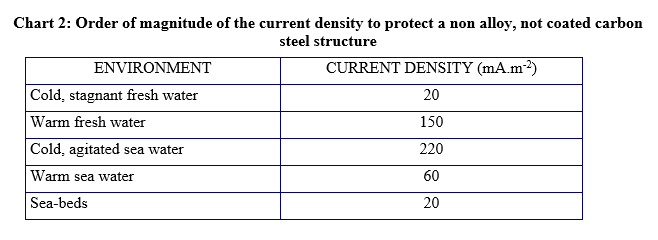
The density of the current which is necessary to obtain cathodic protection depends mainly on the environment surrounding the structure. Chart 2 gives orders of magnitude of this current density for a non-coated steel. The presence of coating enables to decrease this current density, because only part of the steel is put in contact with water (notion of insulation resistance or degradation coefficient).
It should be noted that a steel immersed in sea water gets covered, because of cathodic protection (local pH increase), with a calco-magnesian deposit. This deposit slightly decreases the current density required for protection, after a few months of operation.


