Safety assessment studies for low- and intermediate-level radioactive waste (L/ILW) have shown that Carbon-14 (14C) is one of the major dose-determining radionuclide released from cement-based deep-mined geological repositories [1]. 14C is of specific concern because of its long half-life (5730 yrs), its possibility to be incorporate in the human food chain and the high mobility of the gaseous and dissolved 14C-bearing organic compounds. Currently, very limited information is available on the chemical speciation of 14C-bearing organic compounds. Some studies have shown the predominant formation of oxidized hydrocarbons whereas others reported predominant formation of reduced hydrocarbons during iron corrosion [2]. A complete inventory of the carbon species expected to be formed during iron corrosion is essential for more detailed assessments of the long-term impact of 14C on dose release. To the best of our knowledge, no study has previously determined the production of both oxidized and reduced hydrocarbons in the same corrosion experiment.
Here, batch-type leaching tests were carried out on non-irradiated iron powders in alkaline artificial cement pore water [3] and NaOH solutions [4] in anoxic alkaline conditions. Only a limited number of organic compounds were recorded during corrosion of the iron powder in cement-type alkaline solutions: small oxygenated and reduced organic molecules up to C5, either dissolved in the aqueous phase or enriched in the gas phase [3,4]. Oxygenated compounds (i.e. LMW carboxylates, alcohols and aldehydes) are formed during the exposure of the iron particles to oxic conditions, such as during oxidative pre-treatment. They are retained in the oxide layer of the iron particles but instantaneously released by desorption as soon as the iron powder is in contact with the alkaline solutions (Figure 1) [3,4]. Note that the fraction of oxygenated hydrocarbons released depends on the duration of exposure of the iron powders to oxic conditions [4]. The reduced gaseous molecules (i.e. methane, ethane, ethane, propane, propene, butane) are formed by a Fischer-Tropsch-type mechanism at the surface of iron particles. In contrast to the oxygenated compounds, they are continuously released from the iron surface during the corrosion process (Figure 2) [3,4]. Therefore, their concentrations increase with time due to reducing conditions at the iron-solution interface. On the assumption that the chemical form and reactivity of 12C and 14C are similar at the iron-water interface, the oxidized and reduced small molecules characterized in this study should also be considered as potential 14C-bearing organic compounds released from anoxic corrosion of irradiated steel.
Figures
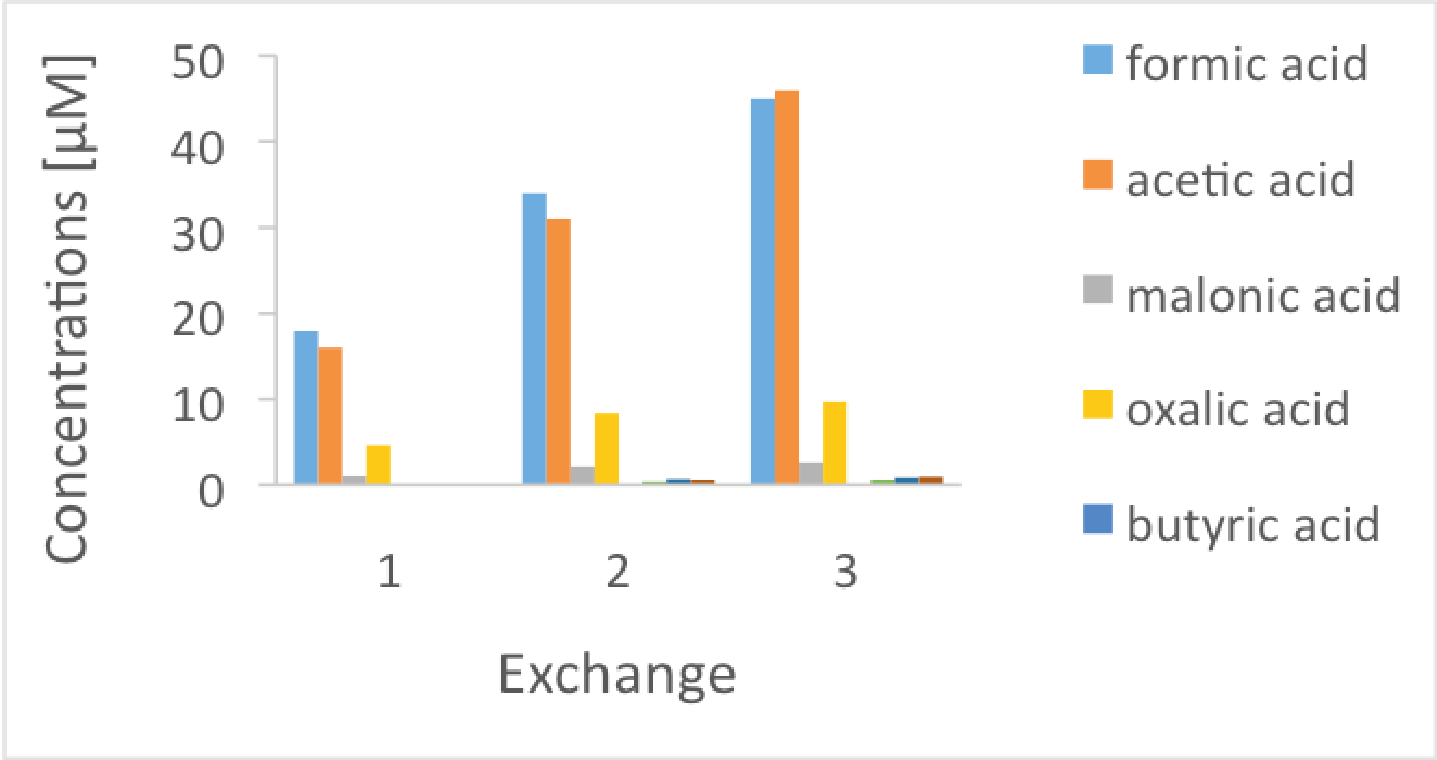
Figure 1: Carboxylic acid release during three consecutives iron powder exchanges. This figure shows the results from corrosion experiments with BASF iron powder that had been oxidized during two weeks and immersed in NaOH solution at pH 12.5 are shown.
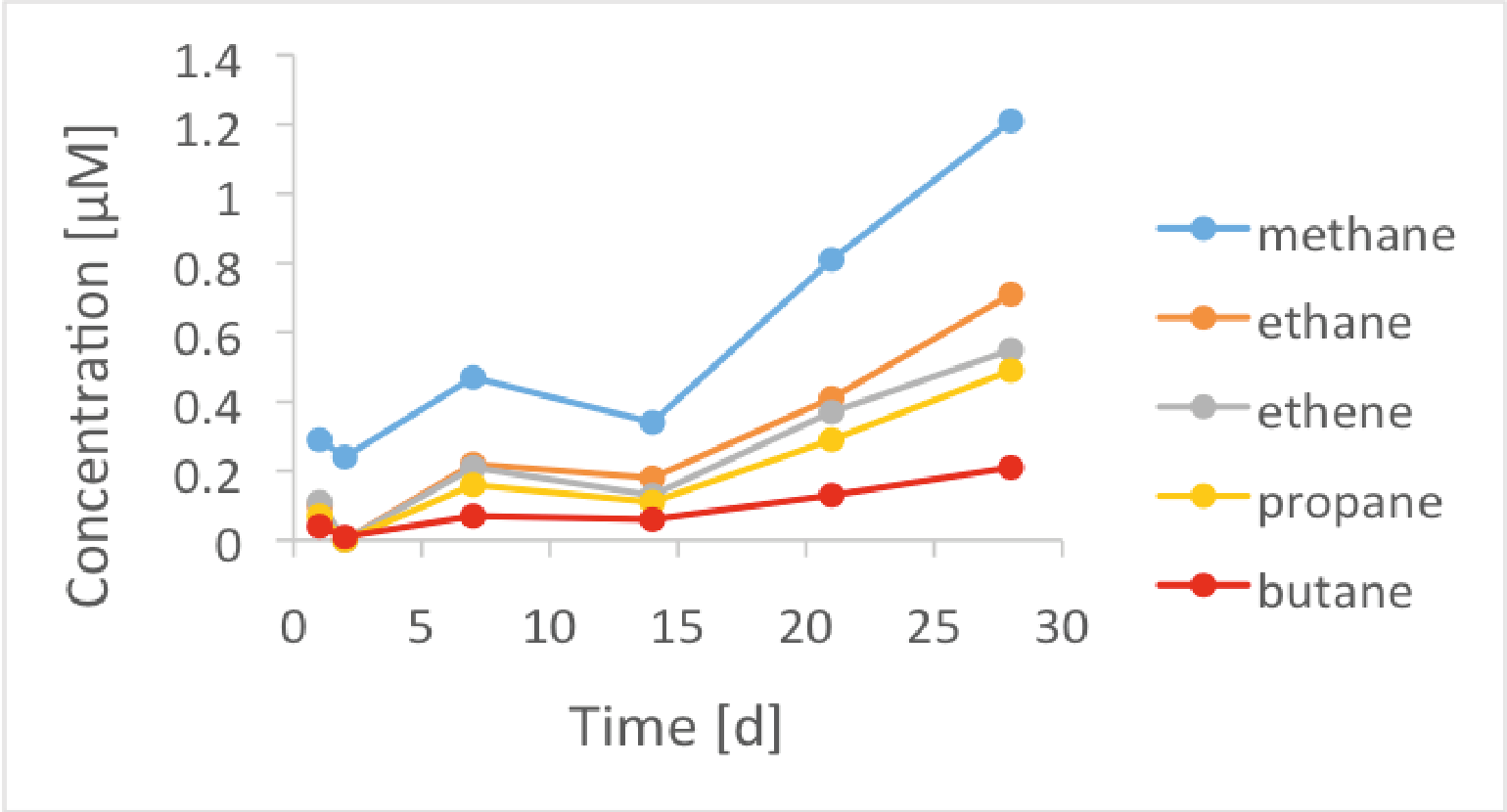
Figure 2: Reduced hydrocarbons formed in a corrosion experiment with BASF iron powder that had been oxidized during two weeks and immersed in a NaOH solution at pH 12.5.
References
[1] Nagra, (2002). Project Opalinus Clay: Safety report. Demonstration of disposal feasibility for spent fuel, vitrified high-level waste and long-lived intermediate-level waste (Entsorgungsnachweis), Nagra, Wettingen, Switzerland.
[2] Wieland, E., Hummel, W. (2015). Formation and stability of carbon-14 containing organic compounds in alkaline iron-water systems: Preliminary assessment based on a literature survey and thermodynamic modelling. Mineral. Mag. 79, 1275-1286.
[3] Cvetković, B.Z., Rothardt, J., Büttler, A., Kunz, D., Schlotterbeck, G., Wieland, E. (2018). Formation of low molecular weight organic compounds during anoxic corrosion of zero-valent iron in alkaline conditions. Environ. Eng. Sci. 35, 437-461.
[4] Guillemot, T., Cvetković, B.Z., Kunz, D., Wieland, E. Batch-type corrosion studies with non-irradiated iron powders in anoxic alkaline conditions. Internal report, Paul Scherrer Institute (in preparation).
iron corrosion; alkaline conditions; radioactive waste management; organic compounds

